After the presentation of the complete line a few weeks ago we are now beginning to discuss in detail about every single test, starting from the fundamental one for marine aquariums, Calcium (qui la recensione in italiano).
ReefStatus Calcium Seachem
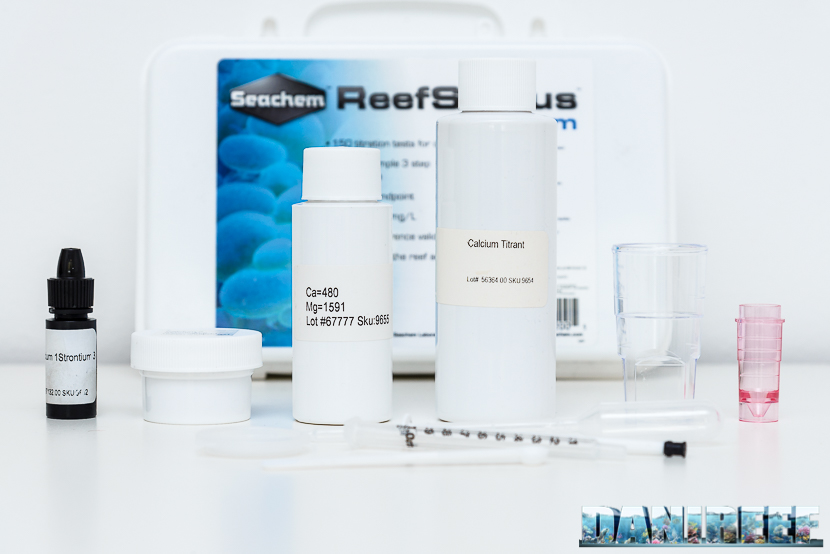
As you can see from the picture the test consists of a plastic container of fine workmanship of big size which contains much material as we see from the photos below. A bottle called “Calcium 1 Strontium 3“, a vial called Calcium Titrant, a container with reagent powder “Calcium 2 Strontium 2“, a small pipe, a 1 ml syringe, a spoon, two transparent plastic containers, one with the cap and one pink. As you can see then the test seems to be packed with the best intentions, even given the cost not just popular of 69,90 euro.
Besides all this, there is also a container with a certain value of the solution of calcium and magnesium, which has to 480 ppm calcium and magnesium to 1591 ppm. We don’t need in this case magnesium, but probably in this way they could prepare a single solution to be included with the two ReefStatus tests. This solution can be used to be sure if the test is aligned before beginning the dosage. It can give us as well an idea if we are doing the test properly and finally we can understand, even after a few months, if the test is still good and reliable.
The test’s resolution is very high and equal to 5 ppm, compared with the tests we have tried in the past, Elos has a resolution of 10 ppm..
Inside the container there is a sponge that allows to hold on all components. In this way they can’t move and beat. We do not know if that is a plus for the test, but for sure it is very useful and attractive.
The test is very laborious and requires more minutes than as usual, unfortunately this is an issue, but it is possible solve it easily if the measured value is accurate.
It fills the pipe up to the mark at base of bulb (wasn’t more easy use a syringe?), And flows into the largest transparent plastic container, the one with transparent color to speak. Approximately it will be 1 ml.
To complete the test it is necessary to use distilled water, or water of excellent quality osmosis, or with TDS equal to zero. You need to fill the pink container to the brim with distilled water, or osmosis.
Fill the osmosis of water into the container with the water to be measured.
Add a drop of reagent “Calcium 1 Strontium 3” in the container. The mixture has to be stirred gently for 30 seconds.
Add 1 scoop of reagent powder “Calcium 2 Strontium 2” to the previous solution, close the container, and shake gently until the reagent is almost completely dissolved.
Fill the syringe up to the value of 1 ml, with the solution called “calcium titrant“,
Then start to dose drop by drop (titration), until the solution will pass from pink color (given by the previous reagents) to blue. It is suggested to shake after every drop to allow the melt solution. When you get to the blue-purple color you will need to add only a further drop.
Now every tenth of a milliliter corresponds to 50 mg/l. Every hundredth of a milliliter, or each notch, would correspond to about 5 ml, with the possibility of estimating by eye even lower values.
If the value of calcium is greater than 500 mg/l, or when you are going to use all the liquid in the syringe, you will have to refill the syringe and then add up the values provided.
As example, as far as it normally affects to us:
- Putting 0.70 ml we will have a calcium value of 350 mg/l, and on the syringe being upside down will read .3;
- Putting 0.75 ml we will have a calcium value of 375 mg/l, and on the syringe being upside down will read .25;
- Putting 0.80 ml we will have a value of calcium 400 mg/l, and on the syringe being upside down will read .2;
- Putting 0.85 ml we will have a calcium value equal to 425 mg/l, and on the syringe being upside down will read .15;
- Putting 0.90 ml we will have a value of calcium of 450 mg/l, and on the syringe being upside down will read .10.
Plus of course all the intermediate scales.
For the Calcium, the reference card does not show a color scale, since you’re talking about a tritrimetric test and there are no predetermined values but only an indication of how to perform the test correctly.